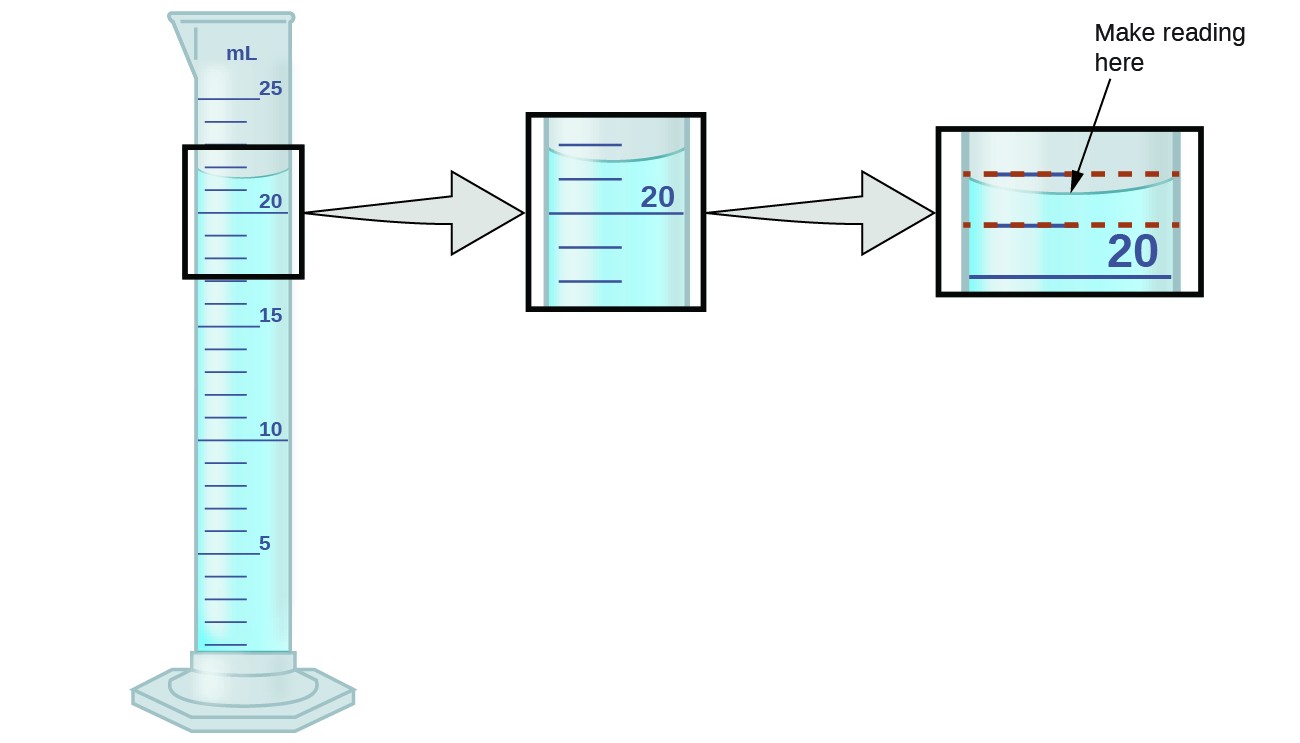
What Is The Uncertainty Of A Beaker . using utmost care, the chemist can only obtain a weight to the uncertainty of the balance or deliver a volume to the uncertainty of the glassware. there is always some level of uncertainty in the measurements that we make in a laboratory, which will affect the precision and accuracy of our results. all measurements have a degree of uncertainty regardless of precision and accuracy. By the end of this section, you will be able to: some of these pieces (e.g. Distinguish exact and uncertain numbers. relative uncertainty •the beaker on the right has a measurement increment of 25 ml •example 2: Beakers, erlenmeyer flasks) are used primarily to hold liquids during experiments. This is caused by two factors,. Upon closer inspection, you will also notice that they,.
from courses.lumenlearning.com
This is caused by two factors,. Upon closer inspection, you will also notice that they,. By the end of this section, you will be able to: Distinguish exact and uncertain numbers. all measurements have a degree of uncertainty regardless of precision and accuracy. using utmost care, the chemist can only obtain a weight to the uncertainty of the balance or deliver a volume to the uncertainty of the glassware. relative uncertainty •the beaker on the right has a measurement increment of 25 ml •example 2: there is always some level of uncertainty in the measurements that we make in a laboratory, which will affect the precision and accuracy of our results. some of these pieces (e.g. Beakers, erlenmeyer flasks) are used primarily to hold liquids during experiments.
Measurement Uncertainty, Accuracy, and Precision Chemistry for Majors
What Is The Uncertainty Of A Beaker all measurements have a degree of uncertainty regardless of precision and accuracy. Beakers, erlenmeyer flasks) are used primarily to hold liquids during experiments. Upon closer inspection, you will also notice that they,. some of these pieces (e.g. using utmost care, the chemist can only obtain a weight to the uncertainty of the balance or deliver a volume to the uncertainty of the glassware. all measurements have a degree of uncertainty regardless of precision and accuracy. relative uncertainty •the beaker on the right has a measurement increment of 25 ml •example 2: there is always some level of uncertainty in the measurements that we make in a laboratory, which will affect the precision and accuracy of our results. Distinguish exact and uncertain numbers. By the end of this section, you will be able to: This is caused by two factors,.
From cemjxopu.blob.core.windows.net
What Is Beaker Used For In The Laboratory at Jeffrey Rodriguez blog What Is The Uncertainty Of A Beaker relative uncertainty •the beaker on the right has a measurement increment of 25 ml •example 2: Beakers, erlenmeyer flasks) are used primarily to hold liquids during experiments. Distinguish exact and uncertain numbers. Upon closer inspection, you will also notice that they,. there is always some level of uncertainty in the measurements that we make in a laboratory, which. What Is The Uncertainty Of A Beaker.
From chem.libretexts.org
1.5 Uncertainty in Measurement Chemistry LibreTexts What Is The Uncertainty Of A Beaker Beakers, erlenmeyer flasks) are used primarily to hold liquids during experiments. all measurements have a degree of uncertainty regardless of precision and accuracy. there is always some level of uncertainty in the measurements that we make in a laboratory, which will affect the precision and accuracy of our results. By the end of this section, you will be. What Is The Uncertainty Of A Beaker.
From www.youtube.com
Precision, Accuracy and Uncertainty in measurement in chemistry YouTube What Is The Uncertainty Of A Beaker Distinguish exact and uncertain numbers. By the end of this section, you will be able to: Beakers, erlenmeyer flasks) are used primarily to hold liquids during experiments. using utmost care, the chemist can only obtain a weight to the uncertainty of the balance or deliver a volume to the uncertainty of the glassware. Upon closer inspection, you will also. What Is The Uncertainty Of A Beaker.
From socratic.org
Why is using a graduated cylinder more accurate than using a beaker What Is The Uncertainty Of A Beaker relative uncertainty •the beaker on the right has a measurement increment of 25 ml •example 2: there is always some level of uncertainty in the measurements that we make in a laboratory, which will affect the precision and accuracy of our results. By the end of this section, you will be able to: Upon closer inspection, you will. What Is The Uncertainty Of A Beaker.
From physics.stackexchange.com
fluid dynamics When the water flows out, will the outlet at the What Is The Uncertainty Of A Beaker using utmost care, the chemist can only obtain a weight to the uncertainty of the balance or deliver a volume to the uncertainty of the glassware. relative uncertainty •the beaker on the right has a measurement increment of 25 ml •example 2: some of these pieces (e.g. By the end of this section, you will be able. What Is The Uncertainty Of A Beaker.
From www.youtube.com
Uncertainty in Measurements YouTube What Is The Uncertainty Of A Beaker By the end of this section, you will be able to: some of these pieces (e.g. there is always some level of uncertainty in the measurements that we make in a laboratory, which will affect the precision and accuracy of our results. Beakers, erlenmeyer flasks) are used primarily to hold liquids during experiments. relative uncertainty •the beaker. What Is The Uncertainty Of A Beaker.
From exoavyrkz.blob.core.windows.net
What Is A Beaker Used For In Chemistry at Kimberly Hickman blog What Is The Uncertainty Of A Beaker relative uncertainty •the beaker on the right has a measurement increment of 25 ml •example 2: Beakers, erlenmeyer flasks) are used primarily to hold liquids during experiments. Upon closer inspection, you will also notice that they,. some of these pieces (e.g. By the end of this section, you will be able to: there is always some level. What Is The Uncertainty Of A Beaker.
From www.youtube.com
read measurement of graduated cylinder beaker and flask YouTube What Is The Uncertainty Of A Beaker Distinguish exact and uncertain numbers. Upon closer inspection, you will also notice that they,. there is always some level of uncertainty in the measurements that we make in a laboratory, which will affect the precision and accuracy of our results. using utmost care, the chemist can only obtain a weight to the uncertainty of the balance or deliver. What Is The Uncertainty Of A Beaker.
From ceivbnmo.blob.core.windows.net
Science Measuring Beaker at Kristine Cooper blog What Is The Uncertainty Of A Beaker Beakers, erlenmeyer flasks) are used primarily to hold liquids during experiments. relative uncertainty •the beaker on the right has a measurement increment of 25 ml •example 2: some of these pieces (e.g. Distinguish exact and uncertain numbers. This is caused by two factors,. using utmost care, the chemist can only obtain a weight to the uncertainty of. What Is The Uncertainty Of A Beaker.
From openoregon.pressbooks.pub
LAB1.3 Measurement Uncertainty, Accuracy, and Precision Introduction What Is The Uncertainty Of A Beaker Upon closer inspection, you will also notice that they,. using utmost care, the chemist can only obtain a weight to the uncertainty of the balance or deliver a volume to the uncertainty of the glassware. relative uncertainty •the beaker on the right has a measurement increment of 25 ml •example 2: some of these pieces (e.g. . What Is The Uncertainty Of A Beaker.
From courses.lumenlearning.com
Measurement Uncertainty, Accuracy, and Precision Chemistry for Majors What Is The Uncertainty Of A Beaker This is caused by two factors,. some of these pieces (e.g. By the end of this section, you will be able to: using utmost care, the chemist can only obtain a weight to the uncertainty of the balance or deliver a volume to the uncertainty of the glassware. all measurements have a degree of uncertainty regardless of. What Is The Uncertainty Of A Beaker.
From www.sciencecompany.com
PYREX 1000250 Glass Beaker, 250mL What Is The Uncertainty Of A Beaker Distinguish exact and uncertain numbers. Upon closer inspection, you will also notice that they,. By the end of this section, you will be able to: using utmost care, the chemist can only obtain a weight to the uncertainty of the balance or deliver a volume to the uncertainty of the glassware. all measurements have a degree of uncertainty. What Is The Uncertainty Of A Beaker.
From byjus.com
Estimate the volume of water in the second beaker. What Is The Uncertainty Of A Beaker some of these pieces (e.g. relative uncertainty •the beaker on the right has a measurement increment of 25 ml •example 2: By the end of this section, you will be able to: using utmost care, the chemist can only obtain a weight to the uncertainty of the balance or deliver a volume to the uncertainty of the. What Is The Uncertainty Of A Beaker.
From www.youtube.com
Online General Chemistry Chapter 1.6 Measurement Uncertainty, Accuracy What Is The Uncertainty Of A Beaker all measurements have a degree of uncertainty regardless of precision and accuracy. This is caused by two factors,. there is always some level of uncertainty in the measurements that we make in a laboratory, which will affect the precision and accuracy of our results. Upon closer inspection, you will also notice that they,. relative uncertainty •the beaker. What Is The Uncertainty Of A Beaker.
From www.youtube.com
How to measure volume with a beaker YouTube What Is The Uncertainty Of A Beaker some of these pieces (e.g. This is caused by two factors,. Beakers, erlenmeyer flasks) are used primarily to hold liquids during experiments. all measurements have a degree of uncertainty regardless of precision and accuracy. relative uncertainty •the beaker on the right has a measurement increment of 25 ml •example 2: using utmost care, the chemist can. What Is The Uncertainty Of A Beaker.
From www.thoughtco.com
The Relative Uncertainty Formula and How to Calculate It What Is The Uncertainty Of A Beaker Beakers, erlenmeyer flasks) are used primarily to hold liquids during experiments. using utmost care, the chemist can only obtain a weight to the uncertainty of the balance or deliver a volume to the uncertainty of the glassware. some of these pieces (e.g. Distinguish exact and uncertain numbers. relative uncertainty •the beaker on the right has a measurement. What Is The Uncertainty Of A Beaker.
From www.chegg.com
Solved Question.2 The beaker and cylinder uncertainty and What Is The Uncertainty Of A Beaker Distinguish exact and uncertain numbers. This is caused by two factors,. using utmost care, the chemist can only obtain a weight to the uncertainty of the balance or deliver a volume to the uncertainty of the glassware. Upon closer inspection, you will also notice that they,. relative uncertainty •the beaker on the right has a measurement increment of. What Is The Uncertainty Of A Beaker.
From www.chegg.com
Solved Observe the contents of the beakers and the dialysis What Is The Uncertainty Of A Beaker Upon closer inspection, you will also notice that they,. relative uncertainty •the beaker on the right has a measurement increment of 25 ml •example 2: there is always some level of uncertainty in the measurements that we make in a laboratory, which will affect the precision and accuracy of our results. This is caused by two factors,. . What Is The Uncertainty Of A Beaker.